Description
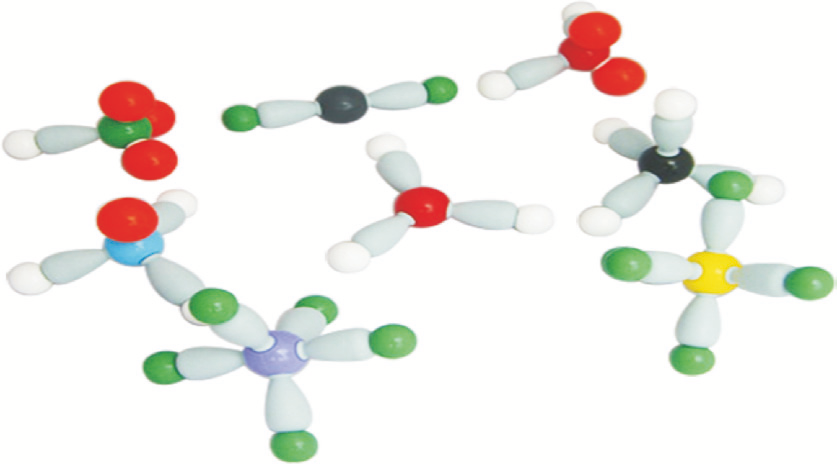
8 model collection set This set contains sufficient parts to make
the eight atomic models shown in the adjacent photograph.
The different shapes are examples of the orientations of the
bonds and cover coordination numbers 1 to 6. Lone pairs are
represented by brown spheres or brown pear shaped parts.
SC6-0256
No. Coord Shape Formula Compound
1 1 Linear HCl Hydrogen chloride
2 2 Linear BeCl2 Beryllium chloride
3 2 Bent H2O Water
4 3 Trigonal planar BH3 Boron trihydride
5 3 Pyramidal NH3 Ammonia
6 4 Tetrahedral CH4 Methane
7 5 Trigonal bipyramidal PCI5 Phosphorus pentachloride
8 6 Octahedral SF6 Sulphur hexafluoride




Reviews
There are no reviews yet.